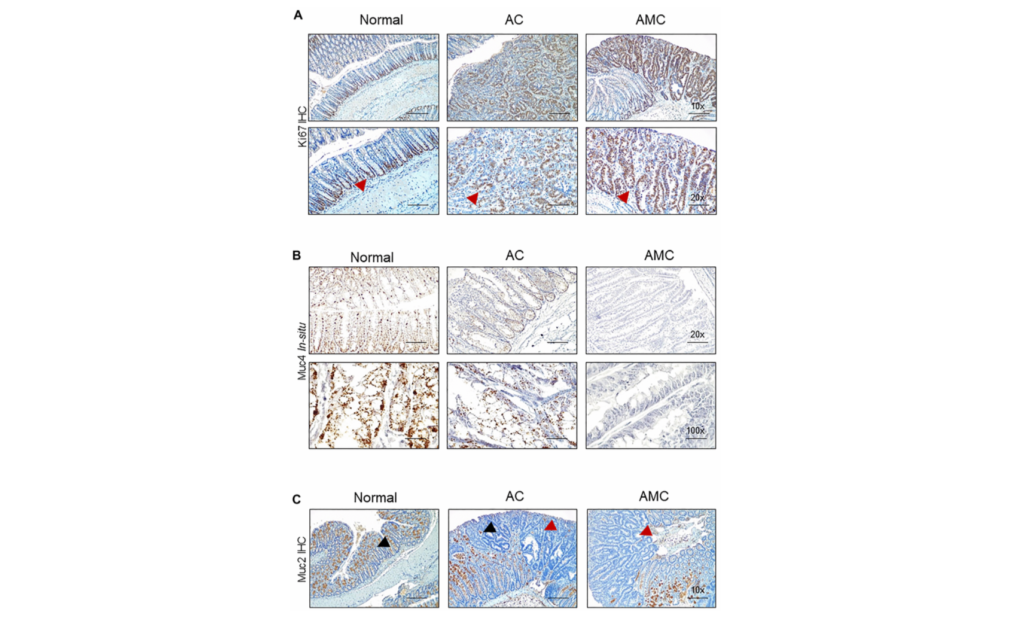
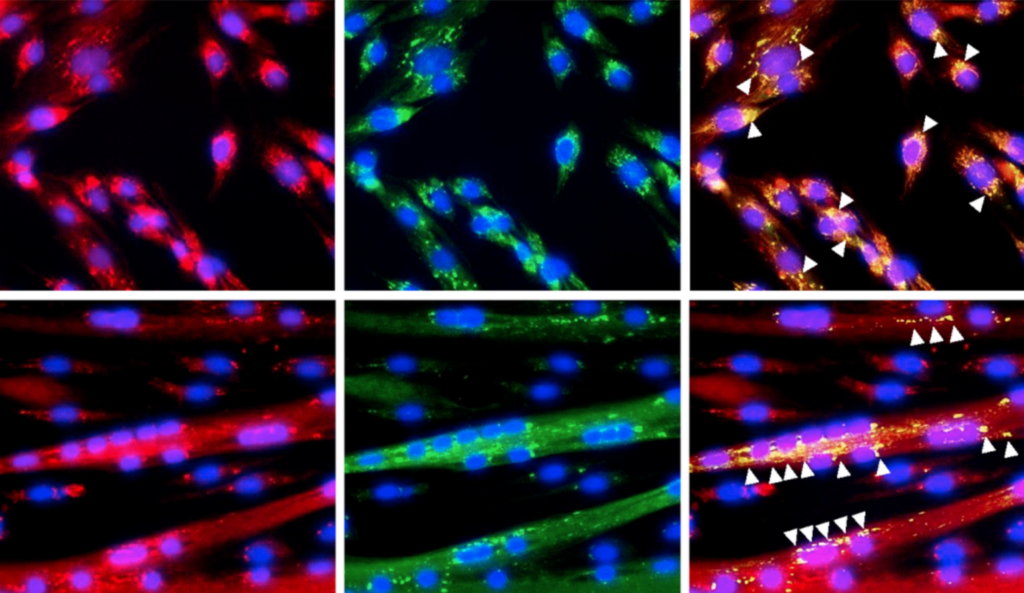
Researchers investigated the mitochondrial protein GRSF1 for its role in the physiology of skeletal muscle aging.
Skeletal muscle is responsible for regulating physical movement and comprises between 30 and 40% of the human body’s mass. The loss of skeletal muscle has major impacts on overall health and quality of life—leading to frailty and a decreased ability to perform activities of daily living. The most common cause of muscle loss is aging, and a prevalent pattern of aging-associated muscular decline is known as sarcopenia.
“With advancing age, the progressive loss of skeletal muscle mass and function, known as sarcopenia, leads to reduced muscle strength and diminishes individual mobility, quality of life, and lifespan [12].”
In a research paper published in Aging (Aging-US) Volume 13, Issue 11, researchers from the National Institutes of Health’s National Institute on Aging and Chungnam National University investigated a protein that may play a role in aging-related muscle loss. Their paper was published on June 2, 2021, and entitled, “GRSF1 deficiency in skeletal muscle reduces endurance in aged mice.”
Skeletal Muscles and Mitochondrial Proteins
The healthy operation of skeletal muscle is dependent on well-regulated mitochondrial functioning. Skeletal muscle is extremely rich in mitochondria, as mitochondria supply muscle cells with the energy they need to help move the body, known as adenosine 5′-triphosphate, or ATP. With age, the mitochondria in skeletal muscles begin to progressively malfunction. The exact mechanisms involved in this decline have not been fully elucidated.
“In aging skeletal muscle, mitochondria display reduced function, altered morphology, and increased production of reactive oxygen species (ROS), which contribute to a progressive loss of muscle mass and strength [13, 14].”
The guanine-rich RNA sequence binding factor 1 (GRSF1) protein is widely distributed in mammalian organs, and primarily enriched in mitochondria organelles. This makes the skeletal muscle an ideal organ in which researchers can study GRSF1, and other mitochondrial proteins, to investigate their role in aging-related processes such as sarcopenia. Although GRSF1 has been well-studied for its role in maintaining mitochondrial function, the involvement of GRSF1 in skeletal muscle aging had not yet been investigated until this study.
The Study
In this study, the researchers used Grsf1cKO mice—a mouse model in which GRSF1 is specifically knocked out in murine skeletal muscle cells. The mice appeared normal until 7-9 months of age. At 16-18 months of age, however, the researchers observed a reduction in muscle endurance compared to wild-type (WT) control mice. The authors postulated that these results suggested the loss of GRSF1 in skeletal muscle may not alter muscle function until later in life.
“The Grsf1cKO mice at this age ran about a 30% shorter treadmill distance on average relative to WT controls (Figure 3A).”
Upon further transcriptomic analysis, the team found that more than 200 muscle genes were differentially expressed in the GRSF1-deficient mice compared to the control mice. Some of the differentially expressed RNAs that were elevated in the Grsf1cKO mice were the hypoxia-inducible Mgarp mRNA, the mRNA encoding Sarcolipin (SLN), the pro-inflammatory proteins CXCL10 and NFKB2, and the transcription factor ATF3. The authors suggested that increased SLN mRNA may also potentially contribute to the decline in skeletal muscle endurance seen in Grsf1cKO mice.
“The reduction of endurance in Grsf1cKO muscle was accompanied by differential expression of several mRNAs, including some that encoded mitochondrial proteins, inflammatory proteins, ion transporters, and transcription factors (Mgarp, Sln, Cxcl10, Nfkb2, and Atf3 mRNAs).”
Conclusion
The researchers found that the absence of GRSF1 in murine skeletal muscle cells led to a decrease in muscle endurance. Initially, the researchers had anticipated that GRSF1 knock-out would lead to a dramatic loss in muscle function. However, their study revealed that the function of GRSF1 in skeletal muscle appeared to only be moderate. Overall, this is an important finding, as it provides new insights into the role of GRSF1 in muscle physiology and opens up new avenues for research into potential therapies for aging-related muscle loss.
“This modest in vivo effect suggests that there are redundant or compensatory mechanisms that prevent catastrophic damage from GRSF1 loss in aging muscle, and that identifying such factors might be of therapeutic benefit in diseases caused by impaired function of muscle mitochondria and impaired muscle regeneration.”
Click here to read the full priority research paper published in Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research. These papers are available at no cost to readers on Aging-us.com. Open-access journals have the power to benefit humanity from the inside out by rapidly disseminating information that may be freely shared with researchers, colleagues, family, and friends around the world.
For media inquiries, please contact media@impactjournals.com.