Researchers examined the effects of thoracic radiation-induced senescent cells on tumor progression, and the role of senotherapeutics to mitigate these effects.
The Trending With Impact series highlights Aging (Aging-US) publications that attract higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Aging-US.com.
—
Radiation therapy is a highly-efficacious inducer of cancer cell death. With this being said, radiation has also previously been shown to cause premature senescence in the lung parenchyma. Senescence in cancer cells was previously only thought of as a mechanism capable of suppressing tumor cell proliferation by halting the cell cycle. However, a growing body of evidence shows that senescent cells may play a pro-tumorigenic role in cancer.
In the tumor microenvironment, the accumulation of senescent cells can become tumorigenic due to a lack of normal tissue stem cells and due to the expression of the senescence-associated secretory phenotype (SASP). SASP expression is when senescent cells secrete high levels of inflammatory cytokines, immune modulators, growth factors, and proteases. In addition to reinforcing senescence, SASP can create a biological environment that is immuno-suppressed and tumor-permissive. Radiation-induced senescence has previously been shown to have negative impacts on cancer patients.
“Cells that have undergone premature senescence due to stress, such as irradiation, are resistant to apoptotic cell death and effectively escape immune surveillance, resulting in their accumulation in tissue over time.”
Recently, researchers from the National Cancer Institute investigated the irradiated lung and the impact of radiation-induced senescent parenchymal cells on tumor growth. They also explored three senotherapeutics, rapamycin, INK-128 and ABT-737, for their potential to mitigate radiation-induced senescence. On February 12, 2022, the team’s priority research paper was published on the cover of Aging (Aging-US) Volume 14, Issue 3, and entitled, “Senescence-associated tumor growth is promoted by 12-Lipoxygenase.”
The Study
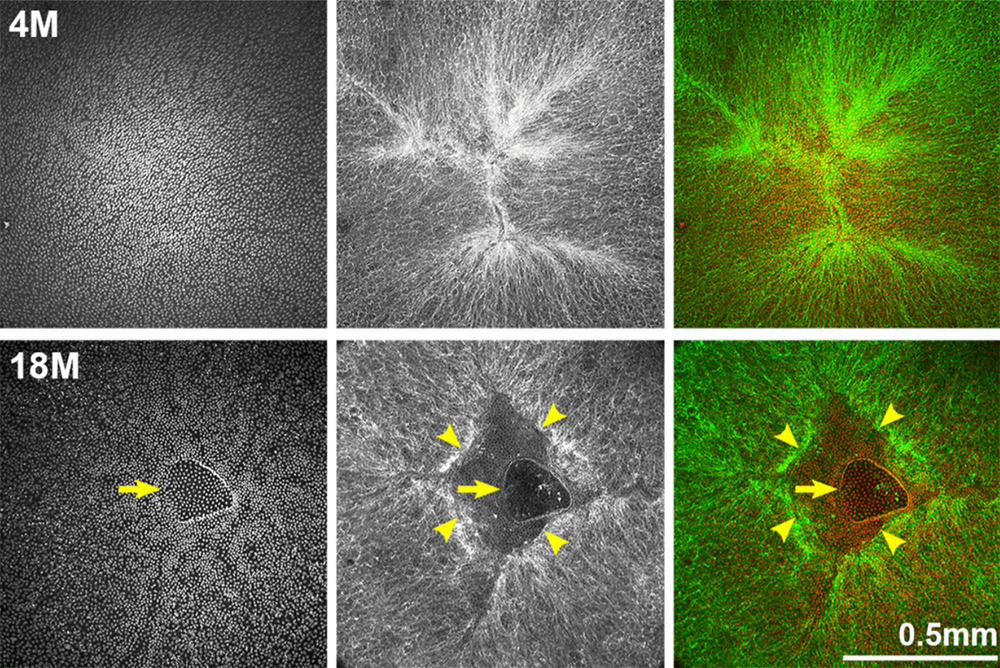
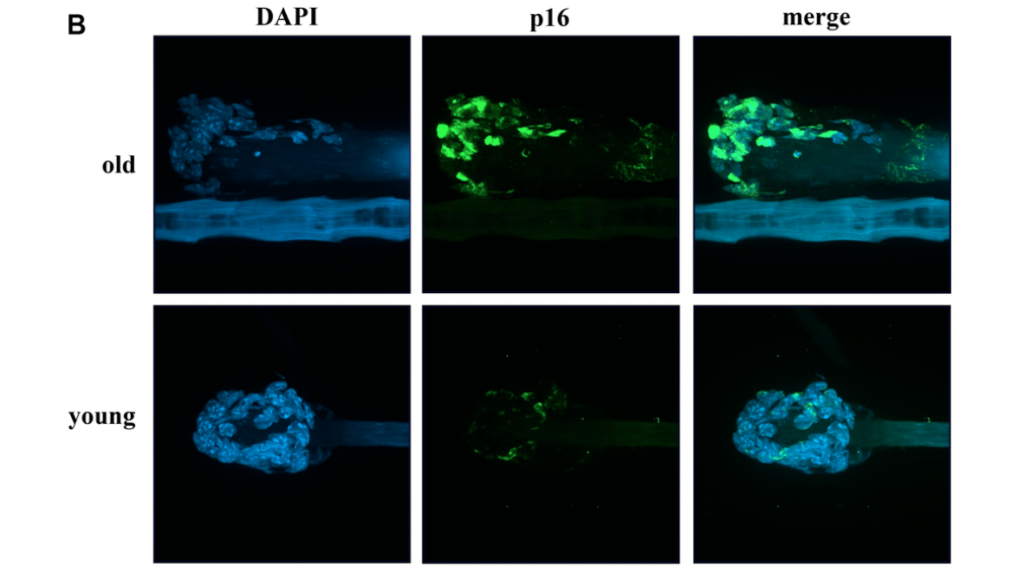
In this study, researchers intravenously injected melanoma cells into murine models two, four and eight weeks after daily fractions of thoracic irradiation exposure. There was also a control arm of unirradiated murine models. Tumor development was monitored by the number and size of the nodules in lung tissues. The number of cells exhibiting senescent activity was also recorded after two, four and eight weeks of thoracic irradiation. Their data demonstrated a correlation between the time points when tumors developed in the irradiated lungs and a marked accumulation of senescent cells.
“As previously described, in irradiated lungs, senescent cells increased significantly 4 and 8 weeks after IR compared to age matched unirradiated controls (Figure 1A).”
A characteristic of oncogene- and stress-induced senescence is the activation of mTOR signaling. Given this connection, the researchers conducted parallel studies evaluating senostatic agents capable of targeting the mTOR pathway, rapamycin and INK-128, and a senolytic agent to selectively eliminate senescent cells, ABT-737. The data showed that rapamycin and INK-128 significantly reduced the number of tumor nodules in the lungs of irradiated mice compared to the controls. ABT-737 demonstrated reduced pulmonary senescence in irradiated mice.
The researchers also studied 12-Lipoxygensae (12-LOX), an enzyme that metabolizes a certain SASP molecule previously implicated in pulmonary senescence: 12(S)-HETE. 12-LOX is a known contributor to radiation-induced senescence and lung injury. The team specifically focused on the role of 12-LOX in pulmonary senescence and its impact on radiation-enhanced tumor growth. They found that inhibiting 12-LOX activity reduced radiation-induced lung senescence and mitigated radiation-enhanced tumor growth.
“Finally, we link senescence associated 12-LOX activity and production of 12(S)-HETE to the observed enhanced tumor growth after irradiation.”
Conclusion
In sum, the researchers found that radiation therapy can induce senescence in the lung parenchyma and also enhance tumor growth. The contribution of senescence in tumor progression was emphasized by the protection delivered by the mTOR-targeted senostatic and senolytic agents. This important discovery could lead to new therapies for cancer patients who are undergoing radiation therapy.
“Together, this study demonstrates the critical role of senescence in mediating radiation-enhanced tumor growth and identifies Alox12 as an important player in this phenomenon. Treatment with a senostatic agent, INK-128, identified in this study, or with agents like rapamycin and ABT-737 suggested their potential therapeutic use in alleviating radiation associated tumor growth.”
Click here to read the full priority research paper published by Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.