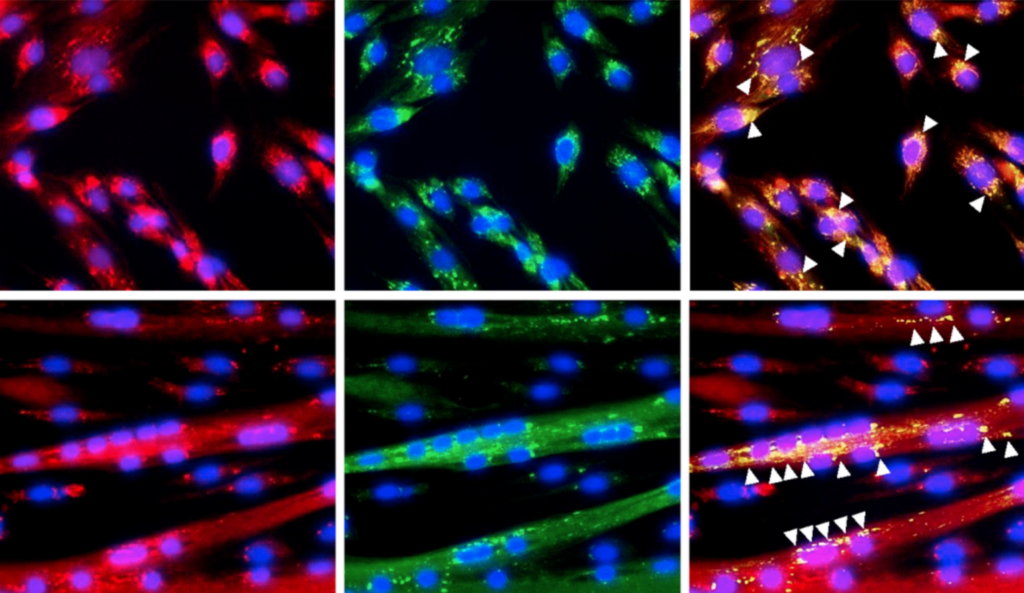
In this new study, researchers aimed to further elucidate the mechanisms of sarcopenia by examining the influence of denervation in young and middle-aged mice.

The Trending With Impact series highlights Aging publications (listed as “Aging (Albany NY)” by Medline/PubMed and “Aging-US” by Web of Science) that attract higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Aging-US.com.
—
A hallmark characteristic of aging is the progressive loss of skeletal muscle mass, known as sarcopenia. A process called motor neuron denervation (Den)—when nerve signals to muscles are blocked or reduced—leads to muscle atrophy, fatigue and eventually muscle loss. Determining how and when Den events influence older muscles is crucially important for developing interventions to stop or reverse age-related muscle wasting.
“Further, aged muscle exhibits reduced plasticity to both enhanced and suppressed contractile activity. It remains unclear when the onset of this blunted response occurs, and how middle-aged muscle adapts to denervation.”
Dysfunctional mitochondria in muscle tissue are known to increase with age. Lysosomes are responsible for the recycling of damaged mitochondria. However, as muscles age, lysosomal function in muscle tissue also declines.
In a new study, researchers Matthew Triolo, Debasmita Bhattacharya and David A. Hood from York University in Toronto, Canada, aimed to characterize the time-dependent changes in denervated skeletal muscle from middle-aged mice. The team focussed on how mitochondrial turnover is impacted. On November 4, 2022, their research paper was published in Aging’s Volume 14, Issue 22, entitled, “Denervation induces mitochondrial decline and exacerbates lysosome dysfunction in middle-aged mice.”
The Study
“The purpose of this study was to compare mitochondrial turnover pathways in young (Y, ~5months) and middle-aged (MA, ~15months) mice, and determine the influence of Den.”
Male mt-Keima mice aged 4-6 months (young) and 14-16 months (middle-aged) were included in this study. The researchers performed surgical procedures to induce Den in the hindlimb muscles of the study mice. After one, three, or seven days of Den, tissue was excised and imaged using confocal microscopy. The researchers collected whole-muscle protein extracts and conducted Western blotting. Statistical analysis was performed using the data they collected.
The middle-aged muscles were compared to muscles from control and young mice. The researchers found that muscle mass, mitochondrial content and PGC-1α protein levels were not different between the young and middle-aged mice. However, indications of enhanced mitochondrial fission and mitophagy and a greater abundance of lysosome proteins were evident in the middle-aged muscle. Their data suggest that increases in fission drive an acceleration of mitophagy in middle-aged murine muscle in order to preserve mitochondrial quality.
“Den exacerbates the aging phenotype by reducing biogenesis in the absence of a change in mitophagy, perhaps limited by lysosomal capacity, leading to an accumulation of dysfunctional mitochondria with an age-related loss of neuromuscular innervation.”
Conclusion
“In our present study, the inability to upregulate mitophagy flux with denervation is driven by a combination of 1) failure to increase mitophagic proteins and 2) the appearance of dysfunctional lysosomes.”
This latest study may shed light on how muscles age and reveal the importance of mitophagy and lysosomal function in maintaining healthy muscles among middle-aged mice. The study also highlights that denervation induces mitochondrial decline and exacerbates lysosome dysfunction in muscles, thereby worsening age-related muscular atrophy. Further studies are needed to gain a deeper understanding of the mechanisms behind these changes and how they can be prevented or reversed.
“Thus, therapies to combat muscle wasting with age-related physiologic denervation must be designed accordingly. Our results imply targeting both mitochondrial biogenesis and maintenance of lysosome capacity will serve to restore mitochondrial homeostasis and likely metabolic capacity of skeletal muscle.”
Click here to read the full research paper published by Aging.
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging is an open-access journal that publishes research papers bi-monthly in all fields of aging research. These papers are available at no cost to readers on Aging-us.com. Open-access journals have the power to benefit humanity from the inside out by rapidly disseminating information that may be freely shared with researchers, colleagues, family, and friends around the world.
For media inquiries, please contact [email protected].