A new device has been developed by researchers to efficiently and painlessly collect hair follicle tissue samples from laboratory mammals, and even humans.
The Trending With Impact series highlights Aging (Aging-US) publications that attract higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Aging-US.com.
—
Laboratory mammals have impacted human-kind far beyond enhancing scientific knowledge in behavioral and environmental research. These animals have greatly contributed to human healthspan and lifespan in countless ways; from validating life-saving cancer therapies to accelerating the future of human anti-aging and longevity interventions. With respect for these salubrious animals, ethical standards (per country) require that researchers handle laboratory mammals with care, and that pain and stress are minimized. Blood and skin tissue samples (biopsies) collected from animals should be replaced whenever possible. For the researchers, this twofold invasive procedure for the animals is also time- and resource-limiting—presenting a bottleneck in the biomedical research process.
“However, we present here a simple method for obtaining biological material in the form of follicular cells from laboratory mice with sufficient quantities and quality for multiple analyses using standard modern molecular biology methods.”
In an effort to efficiently and humanely solve this ethics/logistics problem, researchers—from Palacky University, University Hospital Olomouc, Danish Cancer Society Research Center, and Karolinska Institute—developed a novel, non-invasive device that can be used to collect tissue samples from hair follicles. They tested the applications of this device and authored a research paper of the study. In December of 2021, their paper was published on the cover of Aging (Aging-US) Volume 13, Issue 23, and entitled, “An efficient, non-invasive approach for in-vivo sampling of hair follicles: design and applications in monitoring DNA damage and aging.”
“As millions of laboratory mice are routinely genotyped globally every year this approach represents a major ethical and logistic breakthrough.”
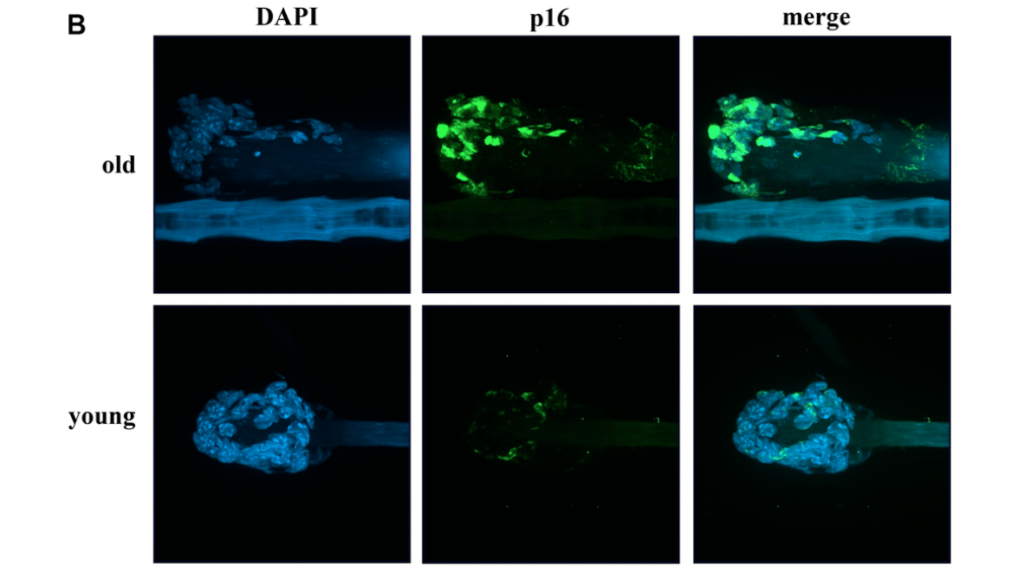
The Follicular Cells’ Collector
As opposed to traditional biopsies, hair follicle collection is a humane, easy, non-invasive, and painless method of DNA and tissue sample collection. Each hair follicle contains approximately 50 cells—of various cell types.
“This micro-organ structure also has other advantages in biomarker studies, including suitability for investigations of circadian rhythms [5, 7], and the presence of numerous cell types in a small area, which can be easily distinguished, such as keratinocytes, melanocytes, or perifollicular macrophages and mast cells [8–10].”
Previously, the limitations of using hair follicles as DNA and tissue samples stemmed from ineffective technology. Many devices involved ordinary tweezers and forceps with high risks for cross-contamination. The researchers termed their novel tissue sample collection device the “follicular cells’ collector.” The follicular cells’ collector is designed with dual pipettes and utilizes a precision vacuum method of hair follicle extraction. The device can be used to comfortably collect DNA and tissue samples from laboratory mammals, and even from humans.
“Although hair samples have been previously used for that purpose [29–31], our sample collection approach may motivate researchers to use them more routinely and widely.”
The Study
To validate that these hair follicle samples contain the required genetic information necessary in most studies, researchers compared murine genotyping results of 151 tail biopsies and 151 hair samples. In order to determine the ability of these samples to detect changes in expression patterns induced by external factors, the team also observed the DNA damage response in hair follicle cells after gamma irradiation and after the topical application of chemical clastogens. Further exploring its potential application in aging research, researchers assayed expression patterns of selected markers of biological age and senescence in murine hair follicular cells. The researchers conducted many other tests and experiments using murine hair follicular cells in this study.
“The speed by which the samples can be collected and processed (e.g. by fixation) is among the biggest advantages of our solution as it can be performed within seconds. This fact limits any potential underlying cellular responses and additional DDR [DNA damage response] caused by cofounding stressing factors related to the withdrawal process [2].”
Conclusion
The researchers found that the follicular cells’ collector method of obtaining mouse hair follicular cells can be successfully used for genotyping, quantitative polymerase chain reaction testing and quantitative immunofluorescence. They also demonstrated that this method can successfully monitor quality and expression level changes of selected proteins—induced by external factors and during natural or experimentally induced aging.
“Our results highlight the value of hair follicles as biological material for convenient in vivo sampling and processing in both translational research and routine applications, with a broad range of ethical and logistic advantages over currently used biopsy-based approaches.”
Click here to read the full research paper published by Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].