In this 2019 study, researchers examined murine models to determine common age-related eye lens changes that contribute to eventual vision impairment and loss.
A variety of eye disorders can occur as humans age, including age-related macular degeneration, cataracts, presbyopia, glaucoma, dry eyes, and temporal arteritis. These conditions can contribute to vision impairment and even vision loss. Unfortunately, the full gambit of common age-related eye lens changes which contribute to these disorders is not yet fully defined. However, while mice and primates are different species, their eye lenses share common characteristics. This means that studies in murine models regarding age-related eye lens changes may provide a baseline for aging studies on human eye lenses in the future.
“Little is known about the morphological, mechanical, refractive and cellular changes that occur with advanced age in the lens. Mice offer an opportunity to investigate changes in lens morphometrics, stiffness, transparency and refractive properties with age in a relatively shortened period of time.”
To further define common age-related changes in eye lenses, researchers—from The Scripps Research Institute, University of Delaware, Morehouse School of Medicine, Nottingham Trent University, Japan Synchrotron Radiation Research Institute, and Boston University School of Medicine—conducted an extensive study of eye lenses among mice between one and 30 months of age. Their paper was published by Aging (Aging-US) in 2019, and entitled, “Age-related changes in eye lens biomechanics, morphology, refractive index and transparency.”
The Study
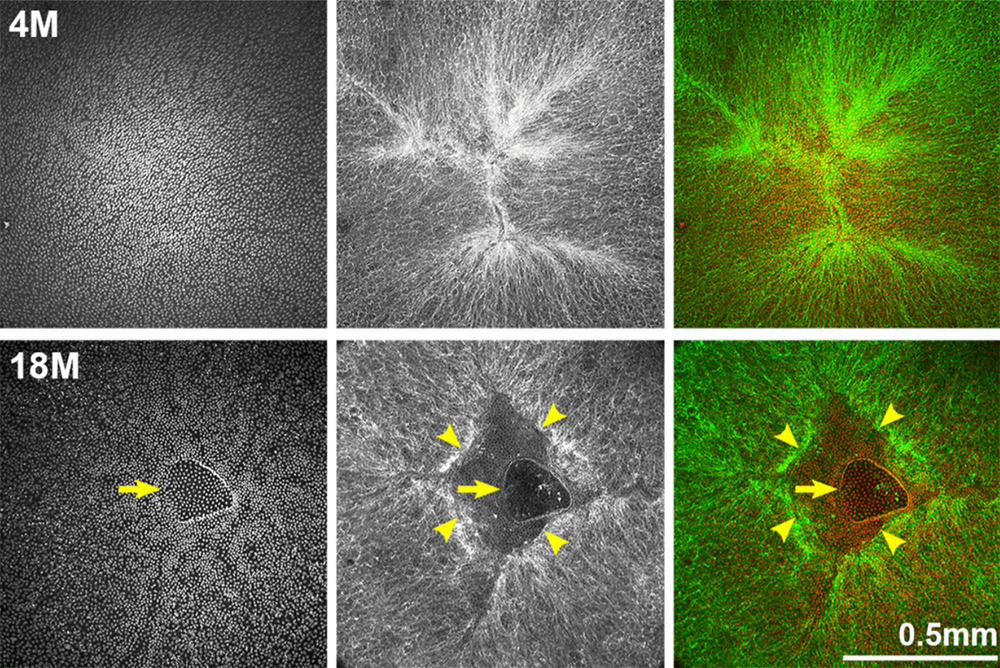
In this study, the researchers measured the size, refractive index (Gradient Refractive Index, GRIN) and stiffness of mouse lenses in young adult mice, starting at one and two months old, to very old mice of 24 to 30 months old. The team examined mechanisms of age-related cataracts, cell morphology in aged lenses, increased lens stiffness with age, and lens resilience. Methods used in this study include: lens biomechanical testing and morphometrics, live lens imaging, capsule thickness and fiber cell width measurements, phalloidin-staining of epithelial cells in whole lenses, scanning electron microscopy, transmission electron microscopy, and X-ray talbot interferometry.
The researchers found that, with age, mouse eye lenses increased in size, nuclear fraction, stiffness, and resilience. After four months of age, lens capsule thickness and fiber cell width did not increase, but epithelial cell area increased slightly with age. In the lenses of mice older than 12 months, the researchers observed anterior cataracts, cortical haziness and ring cataracts. They found that the anterior cataracts were due to incomplete suture closure and detachment of anterior epithelial cells from the underlying fiber cells. The ring cataracts were linked to abnormal compaction of differentiating fiber cells. The hexagonal packing of fiber cells was shown to be disrupted with age. Lastly, the researchers observed that the gradient refractive index increased and then plateaued with age.
“Our comprehensive study of aging in wild-type mouse lenses in the B6 genetic background showed increased stiffness along with appearance of anterior, cortical and ring cataracts with age (Figure 14).”
Conclusion
Overall, the researchers demonstrate that age-related changes in mouse lenses mimic some aspects of aging in human lenses. Aside from the obvious study limitations (mouse-to-human translation), the data collected from this study provide a comprehensive overview of age-related changes in murine lenses, including lens size, stiffness, nuclear fraction, refractive index, transparency, capsule thickness, and cell structure.
“Whether there is a common molecular mechanism that drives changes in all the measured parameters remains unknown, but further biochemical and cell morphology studies will be needed to determine how subcellular aging affects the whole tissue.”
Click here to read the full research paper published by Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].