Aging Editorial Board member Andrei V. Gudkov, PhD, DSci, discusses his 2017 research paper published by Aging, entitled, “p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli.”

Behind the Study is a series of transcribed videos from researchers elaborating on their recent oncology-focused studies published in Aging. A new Behind the Study is released each Monday. Visit the Aging YouTube channel for more insights from outstanding authors.
—
Greetings. My name is Andrei Gudkov. I am working in Roswell Park Cancer Institute, designated cancer center located in Buffalo, New York. I am Senior Vice President for Basic Science and chair of Department of Cell Stress Biology. My research is focused on understanding of the mechanisms of deregulation of a variety of stress response pathways in cancer cells as well as in normal cells in relation to cancer origin, progression, or engraftment and trying to use the information which we are generating during this research to come up with new types of treatment of cancer or cancer prevention.
Recently, our interests have significantly switched towards studying of the mechanisms of aging in its relations to cancer, since, as we all know, both conditions are closely connected. During the last, probably 20 years, one of the central theories of aging in mammals has been evolving towards connection between chronic sterile inflammation, which is accumulating in tissues with age of a mammal, including humans, with systemic decline in regeneration capabilities, in function of organs and tissues, and increasing risk of major diseases, altogether known as aging-related diseases. And the source of this inflammation, its origin, has been the central focus of studies of many.
During last couple of years, the dominating opinion in the field is about the central role of senescent cells, cells which chose to stay irreversibly growth-arrested in response to DNA damage, which they acquire during their life. And, through that, change their phenotype in more significant way than just growth arrest, acquiring the ability to secrete a spectrum of pro-inflammatory factors.
These senescent cells, which initially were defined as such in tissue culture experiments, eventually were proclaimed to be the main suspects in their putative role of inflammation creators in aging organism. This idea has become really popular, especially following a series of brilliant works coming from number of laboratories, in which senescent cells were detected in vivo in mice and mouse models. And when these mice were treated with agents which eradicated these senescent cells, numerous signs of rejuvenation were observed.
I’m talking about the first paper of that kind appeared in 2011, Mayo Clinic, and the group led by Jim Kirkland and Jan van Deursen and a series of follow-up papers with similar results. In general, the idea of putting senescent cells in the position of the key sources of sterile chronic inflammation associated with aging came from Judy Campisi, who has provided the most important discoveries in that field.
Well, this theory is extremely appealing for many reasons. First, it is very well supported by evidence. Indeed, senescent cells, when they turn into senescents in culture, switch their phenotype into, so-called, SASPs, and that’s an associated secretory phenotype, the state in which cells continuously secrete pro-inflammatory factors. Second, these cells appear in culture as a result of serial passaging resembling aging. And, therefore, this link became kind of natural between aging and senescent cells. The presumption was that certain cells in the body who used up the number of divisions they can go through before they reach this state may be increasing with age and, therefore, these cells accumulate.
Each of them may become the source of sterile inflammation. Each single one provides a very weak signal, but, when they accumulate altogether, the impact may become significant and translated into pathological conditions. So recently, there were very few – and, even now, it is like that – very few biomarkers of senescent cells, none of which is very reliable because every single biomarker is kind of promiscuous and is not universally selective for senescent cells.
Among these biomarkers, two have been most popular. One is high level of expression of, so-called, senescent-associated senescent-associated beta-galactosidase, which can be detected chemically in fixed cells and tissues which undergo staining, including X-gal, which turn beta-galactosidase reaction into the blue dye under conditions which is not optimal for endogenous beta-galactosidases mammalian cells at low pH. And, under these conditions, the background beta-gal activity of normal cells is practically not seen and senescent cells become brightly visible. So this reaction, which unfortunately requires a cell… It can not be done on paraffin-embedded sections and require preservation of the enzymatic activity and, therefore, is available, mostly, on the frozen sections or in cells in culture… has been used very, very frequently. And in many papers, it has been just the only assay which was used for detection of so-called senescent cells.
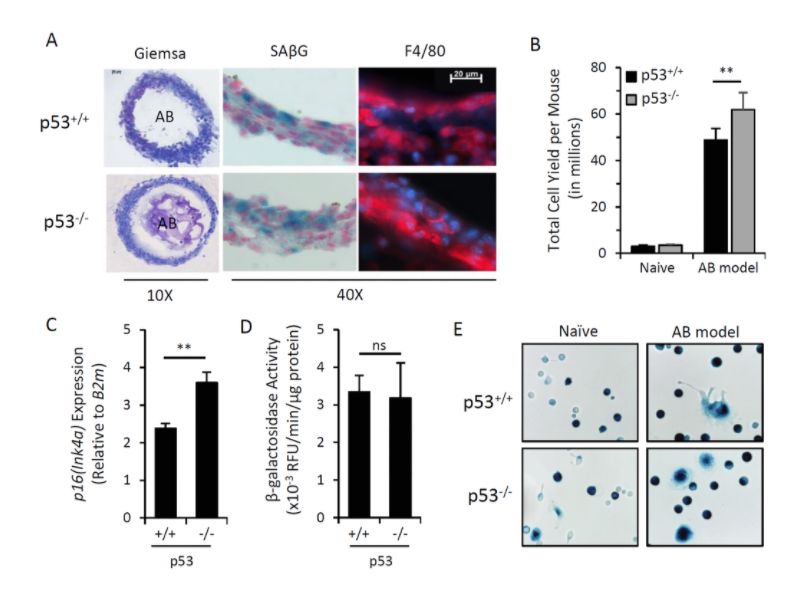
The other biomarker, which resulted from a detailed analysis of promoters which are active selectively in senescent cells is the gene encoding cyclin-dependent kinase inhibitor p16. And the genes name is INK4a. In fact, this promoter of this gene is frequently upregulated in senescent cells, and it has relatively low background in other cells of the organism.
Again, p16 activation is not limited to senescent cells and, moreover, not every senescent cells has elevated p16, but that’s the best we have as of today. That is why, whenever the investigators want to create a mouse model in which they could have the desirable gene expressed exclusively in senescent cells, they use p16 promoter. And there are several mouse models; I’m aware of three in which reported constructs were put under p16 promoter. And the claim was that, when these reporters become obviously expressed in mouse tissues, that was interpreted as accumulation of senescent cells. Also, one can put under this promoter the gene which enables selective eradication of cells with this expression, and, therefore, there is an opportunity to selectively kill such cells. Again, this can be interpreted as a selective eradication of senescent cells.
Using these models, two groups of investigators claim that eradication of senescent cells in aged mice led to substantial demonstration of signs of rejuvenation and, in one case, with increased lifespan. Well, obviously, these data not only provided a very powerful support for the theory about the role of senescent cells in aging but also provided the proof of concept for development of pharmacological approaches to anti-aging treatment and treatment of conditions which lead to the high risk of development of age-related diseases, including cancer.
We obtained such mice in our laboratory, and we have been working with them during last couple of years. The mice we are using are coming from the laboratory of Norman Sharpless from North Carolina. And they have a luciferase reporter gene, which is substituting one of the alleles of p16 and, thereby, being expressed from the p16 promoter. We were pleased to see that these mice accumulate p16-driven luciferase-positive cells detected by in vivo imaging during their lives, which, actually, very well fit the senescent cell theory in their accumulation during life.
However, we were very surprised not seeing accumulation of these cells following total-body radiation or treatment with other genotoxic conditions, which, supposedly, should create lots of senescent cells. We also were puzzled that we were unable to see activation of p16-driven luciferase when we take tissues from these mice and isolated mesenchymal cells from these tissues in vitro and then turn them into senescents, and we failed to see activation of luciferase.
Again, all this together stimulated us to look at the nature of p16-positive cells in these mice and determined their nature, their origin, and their fate in vivo. We started from following the consequences of injection of cells, which would turn into senescents in vitro following injection in vivo into mice. And we labeled cells. We made cell senescents in culture by gum radiation. Then, we injected them intraperitoneal, subcutaneously, into mice. And we looked for their presence by monitoring the label which they were marked with.
Well, it appears that these labeled cells – their traces are disappearing quite quickly, and, within a few days, there are none left in the mice. However, if you put normal cells of similar origin, they actually last much longer. That was the first indication that there may be a mechanism of selective eradication of senescent cells in the body. To check this mechanism and one of our hypotheses was that this mechanism is associated with physical attack of some cells of immunity against senescent cells, and there’s supposed to be innate immunity because it’s happening immediately without any education over the organism.
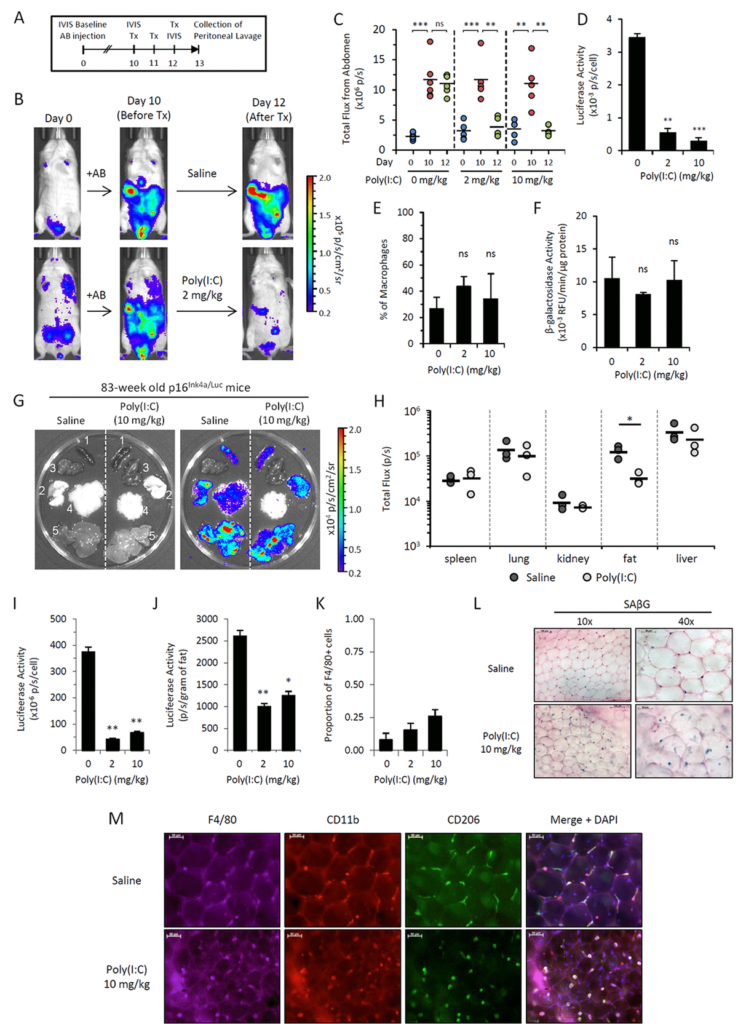
We use a trick in which we embedded senescent cells created in vitro into algenate beads, small spheres consisting of a polymer, which enables to keep cells alive inside them, does not interfere with acquisition of nutrients and oxygen by the cells, but prevents any attack against the cells from any immunocytes. When we took these beads filled with senescent cells and put them in peritoneal cavity of mice, we were pleased to see that they are lasting four weeks without significant death, indicating that senescent cells, who disappear if they are injected without protective beads, are indeed killed by some, so far, unknown mechanism.
In order to identify the executors of senescent cells, we put these beads filled with senescent cells as bait inside, very peritoneal cavity of normal mice, and two weeks later, we pulled them out and analyzed who was accumulating in terms of how cells around these beads in lavage liquid, as well as in the capsule, which was formed around every bead.
Our results brought us to a very important and quite striking observational. The major part of the cells, which was so in these beads as well as in the lavage, appeared to be cells with macrophageal markers on them, which appeared to be bright fluorescence, meaning that they have activated p16, and also positive for beta-gal staining conducted under conditions we are using to reveal senescent cells. So we had to conclude that senescent cells put in the beads attract, probably, by the products of their secretion special subtypes of immune cells, significant proportion of which become reprogrammed to start expressing two biomarkers which people have been using to distinguish senescent cells.
We studied these macrophages in detail, and, after we published our first paper in which we describe this phenomenon, we published a second one, also in Aging, where their properties were described in further details. And we confident that these are bonafide macrophages, not only because they have have biomarkers, they have surface antigen specific for macrophages, but also they are capable of phagocytosis and, moreover, they can be selectively killed by liposome-embedded clodronate, a poison which only kills cells capable of phagocytosis. This killing could be done both in vitro and in vivo when you inject liposomal clodronate inside mice.
So, as far as the presence of these cells in the body of those mice which are not embedded with algenate beads with senescent cells, today, we are confident that these macrophages are accumulating in subcutaneous fat of aged mice in large numbers. And, again, they express biomarkers of macrophages that can be selectively eradicated by clodronate.
So, altogether, it means that the cells which become p16-positive vivo, not necessarily our senescent cells – our operations does not disprove that the signal which we and other investigators are seeing in these mice and increasing with age is not associated with senescent cells. So, potentially, certain proportion of cells we see are, indeed, senescent. However, we are confident that significant part of the signal goes from macrophages, which can be induced into the phenotype associated with expression of both senescent markers when they’re exposed to senescent cells. What is also interesting that this phenotype is reversible. And, in our second paper, we provide a number of physiological stimuli which can either stimulate or suppressed acquisition of this phenotype by macrophages.
All this, together, provides a very interesting step forward in evolution of the theory of aging associated with accumulation of certain specific cell types, contributing to the sterile inflammation occurring in tissues. Today, we can say that those cells which we claim to be the main source of that are not necessarily senescent, but also can be immunocytes who share with senescent cells some of their properties but are not senescent by nature and simply reprogrammed macrophages.
What is the relative impact of these macrophages versus senescent cells towards the process of aging is a very important question, not only from a theoretical standpoint, but also from practical standpoint because, from the time when senescent cells were claimed to be the key players of aging, there have been a substantial effort in the field in generating and testing senolytic compounds, drugs, emerging drugs, which potentially can have anti-aging effect due to eradication of senescent cells from the body.
Whether senolytic compounds would, indeed, solve the issue because, presumably, they will eliminate only a part of the p16-positive cells. To what extent, we need to redirect our attention to the senescent cell-associated macrophages as potential alternative source of secreted factors is an open question. And these are the questions which we are trying to address in our ongoing work, which stems from these observations. Thank you.
Click here to read the full study published in Aging.
—
Aging is an open-access journal that publishes research papers monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].
