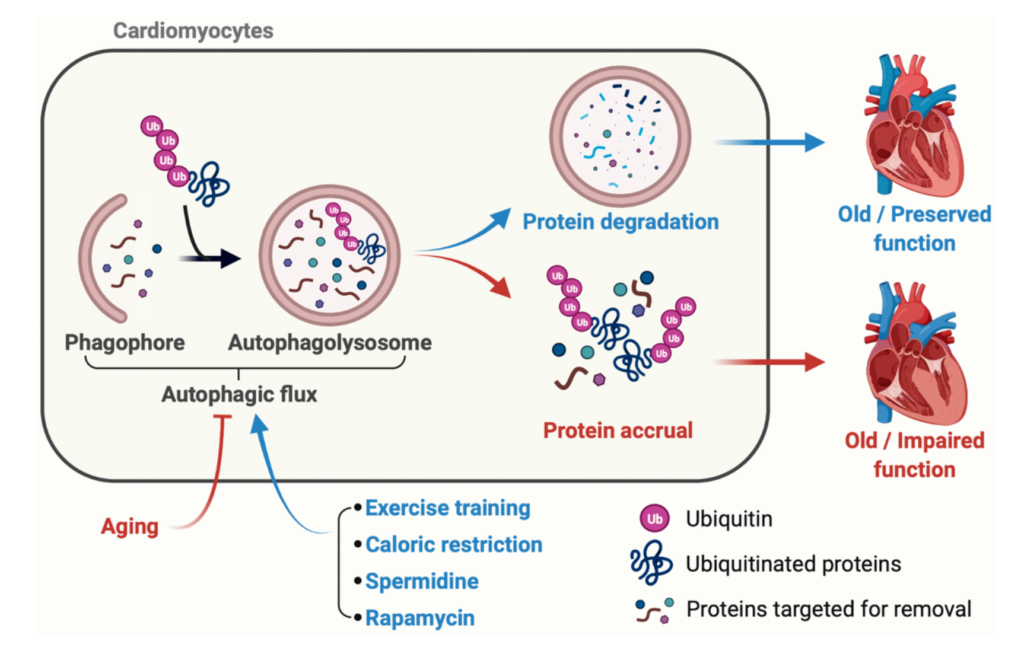
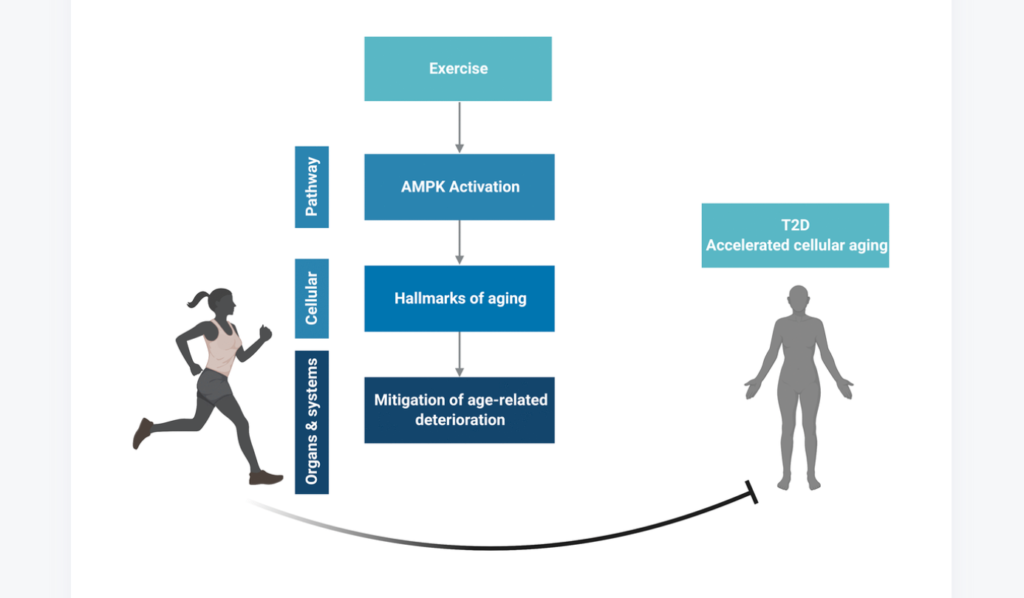
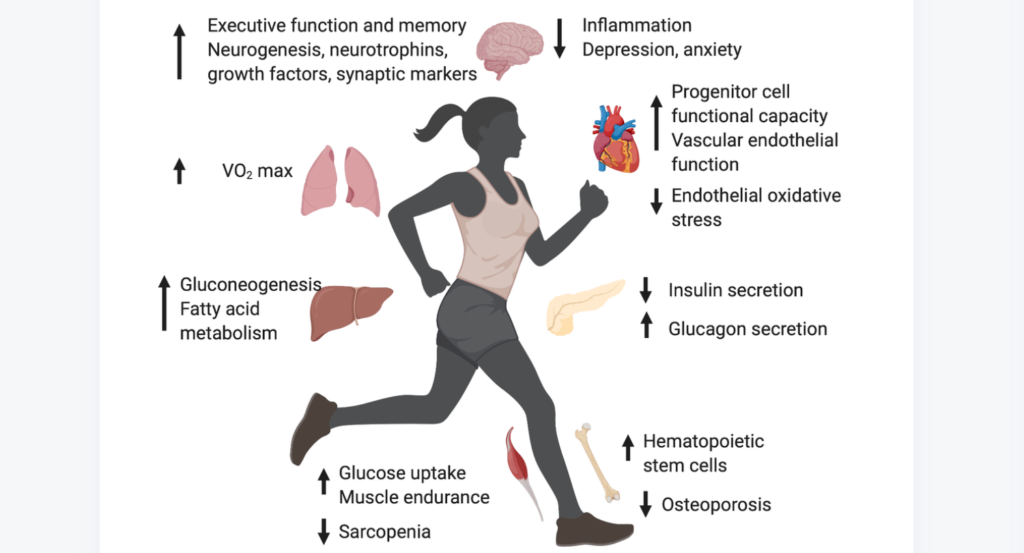
In a new study, researchers investigated myocyte-secreted factors with the potential to suppress cellular senescence, aiming to explore their protective effects against lung disease.
—
Over the human lifespan, our cells encounter numerous stressors that can trigger an intrinsic defense mechanism called cellular senescence. Cellular senescence is characterized by irreversible growth arrest and can act as a safeguard against cancer. However, when senescent cells accumulate in various tissues as we age, it can contribute to tissue degeneration and chronic diseases.
The senescence-associated secretory phenotype (SASP), a hallmark of senescent cells, plays a critical role by secreting inflammatory factors, proteases, and growth factors, disrupting tissue balance and fueling pathological conditions. Consequently, selectively eliminating senescent cells has emerged as a promising therapeutic strategy, potentially restoring tissue function and mitigating age-related disorders.
COPD: Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) exemplifies the impact of cellular senescence on health, characterized by the collapse of alveolar walls in the lungs. Accelerated accumulation of senescent cells in COPD patients’ lung tissues links senescence to the disease’s pathogenesis. Genetic or pharmacological elimination of these cells in preclinical models has shown significant reductions in emphysema-associated pathologies and restoration of pulmonary function, highlighting the potential of senolytic therapies.
Regular physical activity offers benefits beyond fitness, including cardiovascular and mental well-being enhancements, and modulates cellular senescence. Studies show an association between habitual exercise and lower levels of senescence markers in various tissues. Researchers have focused on myokines, signaling factors secreted by skeletal muscles in response to exercise, as potential mediators of these benefits. Irisin, a myokine, has shown promise in suppressing cellular senescence and correlating inversely with COPD severity.
In a new study, researchers Hiromichi Tsushima, Hirobumi Tada, Azusa Asai, Mikako Hirose, Tohru Hosoyama, Atsushi Watanabe, Taro Murakami, and Masataka Sugimoto from Tokyo Metropolitan Institute for Geriatrics and Gerontology, Shigakkan University, and National Center for Geriatrics and Gerontology investigated myocyte-secreted factors with the potential to suppress cellular senescence, aiming to explore their protective effects against lung disease. Their research paper was published on the cover of Aging’s Volume 16, Issue 13, entitled, “Roles of pigment epithelium-derived factor in exercise-induced suppression of senescence and its impact on lung pathology in mice.”
PEDF: A Promising Senescence Suppressor
In this recent study, pigment epithelium-derived factor (PEDF) emerged as a key player in the interplay between exercise, cellular senescence, and lung pathologies. Initially known for its role in retinal development, PEDF has been linked to cellular senescence modulation, extending the replicative lifespan of fibroblasts and diminishing senescence markers. PEDF mitigates oxidative stress by reducing reactive oxygen species levels and modulates microRNAs, particularly miR-127, implicated in cellular senescence.
“We found that myocyte-derived factors significantly extended the replicative lifespan of fibroblasts, suggesting that myokines mediate the anti-senescence effects of exercise.”
Exercise significantly upregulates PEDF expression in skeletal muscles, correlating with reduced senescence markers and SASP-related genes in the lungs. Recombinant PEDF administration in mice has shown remarkable results, reducing senescence markers and preserving alveolar structure in pulmonary emphysema models, translating into improved pulmonary function. While some preclinical evidence supports PEDF’s therapeutic potential, translating these findings to clinical applications requires rigorous safety and efficacy evaluations. Understanding PEDF’s signaling pathways could unveil new therapeutic targets, and its potential involvement in other age-related disorders warrants further investigation. The interplay between PEDF and other exercise-induced factors offers potential for novel therapeutic strategies.
“Collectively, these results strongly suggest that PEDF contributes to the beneficial effects of exercise, potentially suppressing cellular senescence and its associated pathologies.”
Conclusions
The discovery of PEDF’s role in exercise-induced senescence suppression and its therapeutic potential in lung pathologies represents a paradigm shift in senescence research. Understanding the interplay between physical activity, myokine signaling, and senescence modulation can lead to targeted interventions promoting healthy aging. Multidisciplinary collaborations are essential to harness the potential of PEDF and other senescence-modulating factors, paving the way for innovative treatments that alleviate age-related diseases and improve quality of life.
Read the full research paper, published in Aging.
—
Aging is an open-access, traditional, peer-reviewed journal that publishes high-impact papers in all fields of aging research. All papers are available to readers (at no cost and free of subscription barriers) in bi-monthly issues at Aging-US.com.
Click here to subscribe to Aging publication updates.
For media inquiries, please contact [email protected].