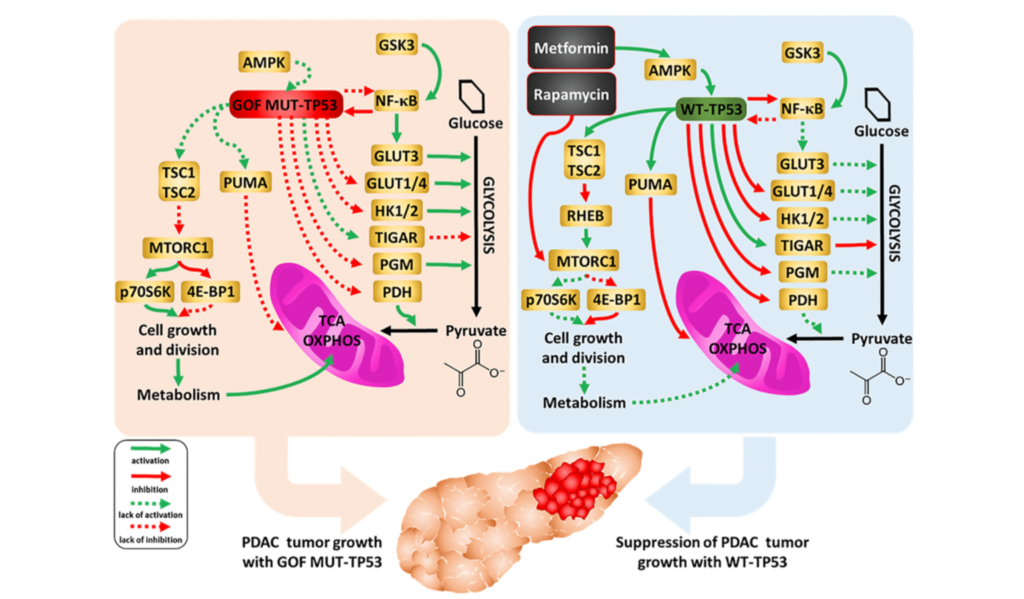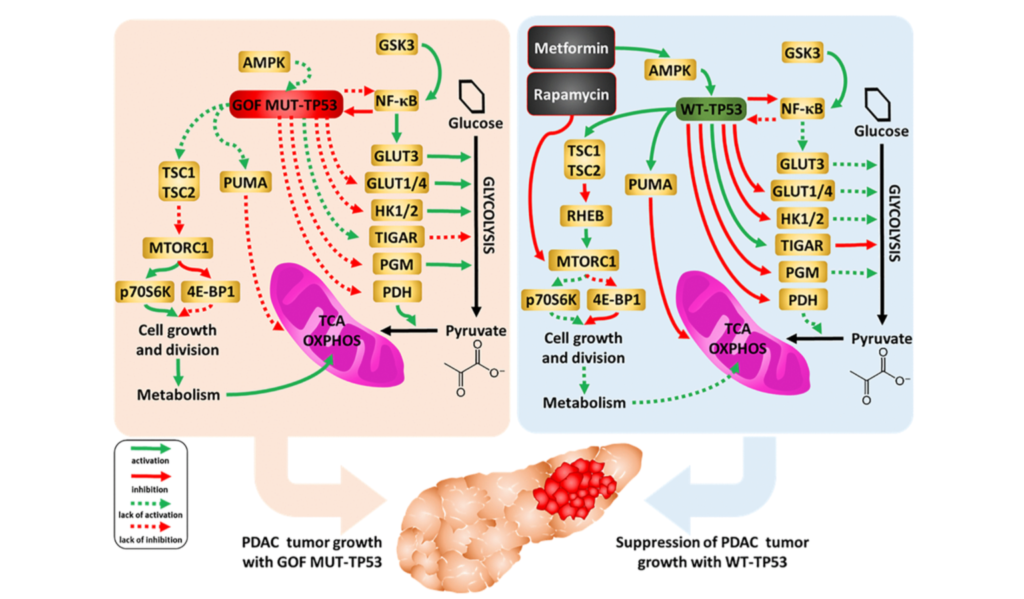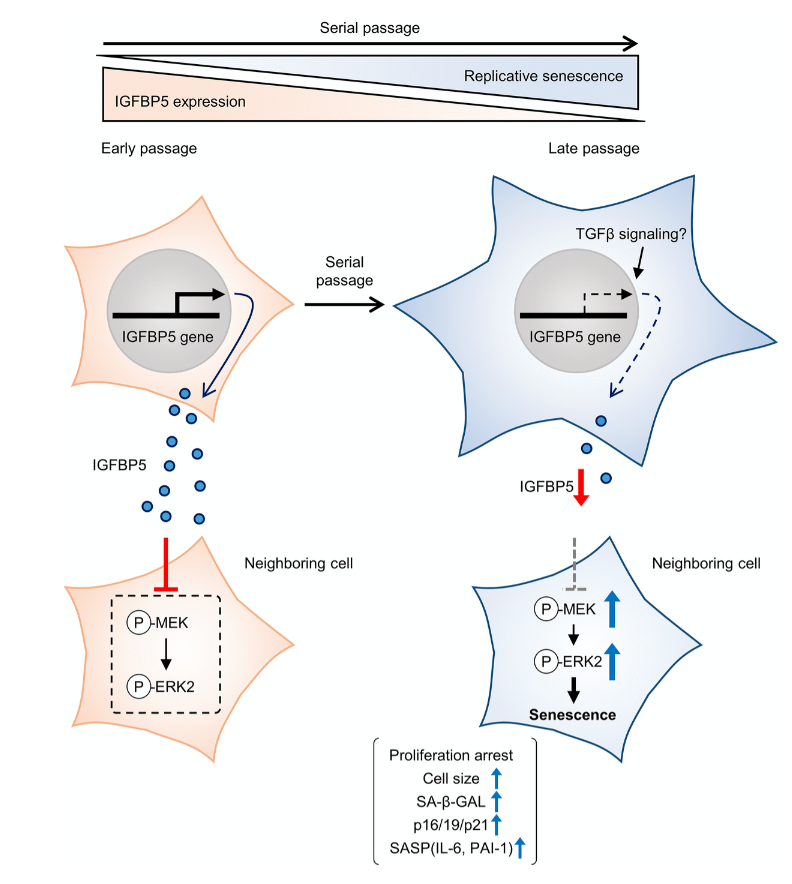
Humans battle a number of biological processes with age that lead to the gradual deterioration of cells and tissues. Frailty, disability, disease, and death are all costly fates of aging. Researchers who study aging aim to change this fate, however, the mechanisms of aging are still all but fully understood.
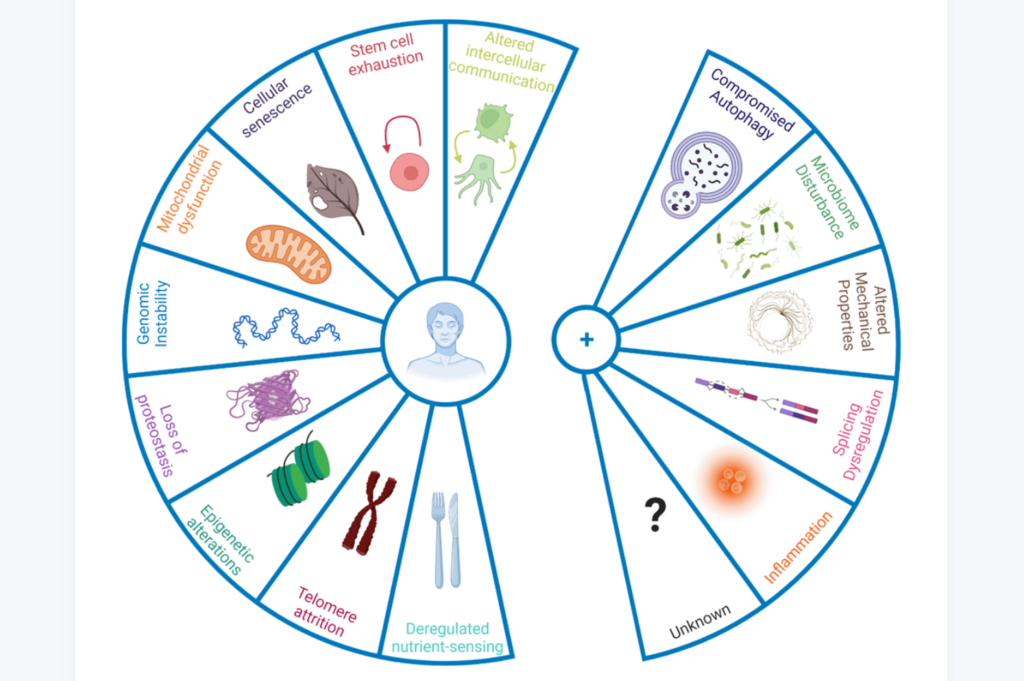
In 2013, López-Otín and colleagues attempted to identify these biological processes and proposed the original nine hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, deregulated nutrient-sensing, cellular senescence, stem cell exhaustion, and altered intercellular communication. These hallmarks of aging have helped to provide a framework for thought about the causes and consequences of aging, as well as potential targets for therapeutic interventions. Now, nine years later, the hallmarks of aging have been updated in light of recent discoveries.
“In the nearly past 10 years, our in-depth exploration on ageing research has enabled us to formulate new hallmarks of ageing which are compromised autophagy, microbiome disturbance, altered mechanical properties, splicing dysregulation, and inflammation, among other emerging ones.”
The “New Hallmarks of Ageing” 2022 Symposium
This update was presented on March 22, 2022, at the “New Hallmarks of Ageing” research symposium in Copenhagen, Denmark. On August 29, 2022, a review paper summarizing the symposium was published in Aging (Aging-US), entitled, “New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary.” The authors of this review are researchers Tomas Schmauck-Medina, Adrian Molière, Sofie Lautrup, Jianying Zhang, Stefan Chlopicki, Helena Borland Madsen, Shuqin Cao, Casper Soendenbroe, Els Mansell, Mark Bitsch Vestergaard, Zhiquan Li, Yosef Shiloh, Patricia L. Opresko, Jean-Marc Egly, Thomas Kirkwood, Eric Verdin, Vilhelm A. Bohr, Lynne S. Cox, Tinna Stevnsner, Lene Juel Rasmussen, and Evandro F. Fang from University of Oslo, Akershus University Hospital, Jagiellonian University, University of Copenhagen, Copenhagen University Hospital Rigshospitalet, Bispebjerg and Frederiksberg, Lund University, University College London, Tel Aviv University, University of Pittsburgh, University of Strasbourg, National Taiwan University, Newcastle University, Buck Institute for Research on Aging, National Institute on Aging, University of Oxford, Aarhus University, The Norwegian Centre on Healthy Ageing (NO-Age), and UPMC Hillman Cancer Center.
Among the keynote speakers who presented at this symposium was Vilhelm A. Bohr, M.D., Ph.D., Chief of the Laboratory of Molecular Gerontology at The National Institute on Aging and a distinguished member of the Aging Editorial Board. Dr. Bohr presented new data on the DNA damage response enzyme poly ADP-ribose polymerase 1 (PARP1)-related pathways. He suggested that PARP1 might be present and functional in mitochondria. Dr. Bohr also presented on the short-term use of NAD+ supplementation in age-related hearing loss.
“Here, Professor Bohr’s group showed that the treatment of mice with NR [nicotinamide riboside] was capable of restoring NAD+ in the cochlea of aged mice to the levels found in young mice. Even more strikingly, NR treatment limited the progression of hearing loss in ageing mice while stopping the progression of hearing loss in old mice.”
The Meeting Report
The authors’ review summarized all the work presented in this symposium, consisting of some of the latest findings in the field and contextualized by the updated hallmarks of aging. Their summaries of presentations were grouped by theme, including theories of aging and cellular senescence, new insights into telomeres and cellular senescence, inflammation, NAD+ and aging, mitochondrial dysfunction, premature aging and DNA repair, and cardiovascular, cerebrovascular and muscular pathologies of aging.
“The presented data showcased novel research at the forefront of the field, with a focus on a possible increase in healthspan and the amelioration of age-related diseases. Here, both the possible clearance or delay of senescent cells as well as possible interventions in the NAD+ system were discussed. While both of these approaches are promising, they are not without limitations.”
A panel discussion took place at the end of the symposium which was moderated by Eric Verdin, M.D., President and CEO of the Buck Institute for Research on Aging and also a distinguished member of the Aging Editorial Board. In sum, the symposium hosted several established researchers and young scientists who presented and discussed the latest findings in age-related research. They discussed new aging research in concert with how it connects to the old and new hallmarks of aging.
“Amalgamation of the ‘old’ and ‘new’ hallmarks of ageing may provide a more comprehensive explanation of ageing and age-related diseases, shedding light on interventional and therapeutic studies to achieve healthy, happy, and productive lives in the elderly.”
Conclusion
The goal of the “New Hallmarks of Ageing” symposium was to provide a platform for researchers to discuss the latest findings in aging research and to update the hallmarks of aging in light of new discoveries. While many promising discoveries have been made in the last decade, the authors cautioned that more work is needed to better understand aging and how these findings can be translated into therapies that improve human healthspan and quality of life. Discussions and research shared at this symposium have the potential to lead to new insights and breakthroughs in the field of aging research.
“At this point, tremendous progress has occurred, but a unified theory of ageing that can fully explain the process is still missing, and many open questions remain, both on a cellular and organismal level. Whether it is possible to target the ageing process at its core, or whether a combination of approaches is needed to target the aspects encompassing ageing, remains to be solved in the future.”
Click here to read the full review paper published by Aging.
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging is an open-access journal that publishes research papers bi-monthly in all fields of aging research. These papers are available at no cost to readers on Aging-us.com. Open-access journals have the power to benefit humanity from the inside out by rapidly disseminating information that may be freely shared with researchers, colleagues, family, and friends around the world.
For media inquiries, please contact media@impactjournals.com