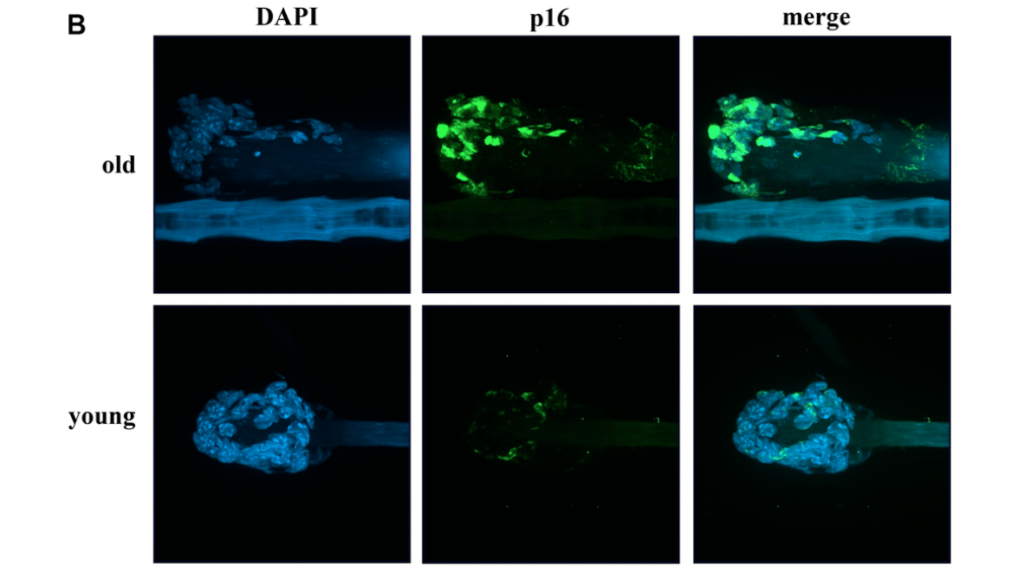
In the cover paper of Aging (Aging-US) Volume 14, Issue 2, researchers discovered a potential therapeutic strategy to target senescent cells and combat aging and age-related diseases.
The Trending With Impact series highlights Aging (Aging-US) publications that attract higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Aging-US.com.
—
Cellular senescence appears to be a phenomenon fundamentally ingrained within the aging process and linked to age-related diseases. Characterized broadly by permanent cessation of the cell cycle, cellular senescence may not be as permanent as once thought.
Researchers from Incheon National University and Korea University conducted a new study exploring analogs of oxazoloquinoline and their potential to alleviate cellular senescence. Their trending research paper was published as the cover of Aging (Aging-US) Volume 14, Issue 2, and entitled, “Targeting regulation of ATP synthase 5 alpha/beta dimerization alleviates senescence.”
THE STUDY
Adenosine triphosphate (ATP) is an energy-carrying molecule found in all living cells. In order to meet the energy demands of the cell, the primary function of the mitochondria is to produce ATP. The maintenance of mitochondrial metabolism is inseparably linked with the regulation of senescence. Therefore, dysfunctional mitochondria has been considered as both a target and the cause of senescence. In addition to a marked decrease in ATP production, senescent cells also increase the expression of inflammatory cytokines, including interleukin 33, or IL-33. The researchers believe that reducing IL-33 may be a possible intervention to reduce senescence in aging patients and age-related diseases.
“In this study, using in-house compound library containing 20 oxazoloquinoline analogs designed to IL-33 inhibitors [9], we aimed to identify compounds capable of ameliorating senescence.”
The researchers investigated 20 oxazoloquinoline analogs using in vitro assays of senescent human diploid fibroblasts and embryonic kidney cells. Efficacy of the candidate compounds was determined using a screening strategy to measure their capacity to increase cell number. Cell numbers were measured between zero and 20 days after compound exposure. The researchers also measured indicators including mitochondrial membrane potential, reactive oxygen species (ROS) levels and p21 expression. They found that the analog KB1541 led to the maximum cell number increase, the recovery of mitochondrial function and the alleviation of cellular senescence. The researchers suggest that KB1541 could be a promising therapeutic agent for use in aging-related diseases.
“The increase in mitochondrial cristae length by KB1541 could be explained by previous findings showing that the increase in ATP generation exerted beneficial effects in mitochondrial function including increases in calcium buffering capacity and decrease in overall ROS production [48].”
CONCLUSIONS
“Taken together, our study provides evidence that the fine-tuning of ATP synthase 5 alpha/beta dimerization by KB1541 can induce mitochondrial functional recovery, concomitant recovery of senescent phenotypes, rendering the use of KB1541 as a potentially advantageous therapeutic strategy in aging and age-related diseases.”
The authors acknowledged that further studies are needed to clarify the exact relationship between IL-33 and mitochondrial energy metabolism. Further studies are also needed to investigate whether other IL-33 inhibitors can modulate senescence by the mechanisms found in the study. This research provides valuable insight into the potential of oxazoloquinoline analogs as novel therapeutic agents for aging and age-related diseases. With further exploration, their findings could lead to new therapeutic strategies to combat aging.
“The role of IL-33 in senescence is not clearly elucidated, therefore discovery of a novel interacting partner will provide clues toward revealing its function.”
Click here to read the full research paper published by Aging (Aging-US).
AGING (AGING-US) VIDEOS: YouTube | LabTube | Aging-US.com
—
Aging (Aging-US) is an open-access journal that publishes research papers bi-monthly in all fields of aging research. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].